In observance of the upcoming holidays, our Customer Support Center will be closed Christmas Eve, Christmas Day, December 26, New Year’s Eve, New Year’s Day and January 2.
Menu
In observance of the upcoming holidays, our Customer Support Center will be closed Christmas Eve, Christmas Day, December 26, New Year’s Eve, New Year’s Day and January 2.
Snoring and obstructive sleep apnea (OSA) are common conditions that dentists can screen for and effectively treat with a custom-made mandibular advancement device (MAD). As dentists look to expand the services offered by their practice and contribute to the patient’s overall health, dental sleep medicine has emerged as one of the fastest-growing areas of dentistry.
However, the traditional medical model of treatment and reimbursement for snoring and OSA can be complex and time-intensive. Dentists consistently cite these challenges as a key limiting factor to the introduction of sleep therapy to their practice.
For 25 years, Glidewell has provided dentists with oral appliances for the treatment of snoring and OSA. More recently, we’ve initiated a provisional mandibular advancement device (PMAD) therapy program, in which we train dentists to easily identify patients who complain of daytime sleepiness or bed partner disturbance due to untreated snoring. Dentists can then offer single-visit sleep treatment using a provisional diagnosis along with our proprietary informed consent form, seeing to the patient’s immediate health need before referral to a physician for follow-up and management.
This streamlined approach offers dentists a simple way to serve their existing patient population and increase practice revenue without the complexity and uncertainty of traditional sleep treatment services.
The provisional mandibular advancement device (PMAD) concept offers dentists a means to simply and reliably treat a vast patient population suffering from snoring and obstructive sleep apnea.
Statistics suggest that up to 50 percent of the patients dentists see every day suffer from sleep-disordered breathing (SDB). The resulting medical conditions can include daytime sleepiness, diabetes, heart disease and stroke.
For dentists starting out in sleep therapy, these are not new patients that have to be recruited, but existing patients who are easily identified if you ask the right questions.
Glidewell’s PMAD program is designed to help dentists successfully incorporate sleep treatment in their practice so that it helps to grow the practice without disruption.



A simple two-part question used to identify patients with sleep-disordered breathing would be: “Do you snore?” If the answer is yes: “Would you like to stop?” This question can easily become part of the everyday patient screening process, undertaken at routine checkups and hygiene appointments.
Patients are accustomed to receiving oral appliances like night guards in the dental office. If they are a candidate for sleep treatment, explain that a sleep appliance is essentially an upper and lower night guard joined by hinges, straps, or other connectors that help to gently reposition the mandible forward to help open up the airway. It’s a subtle change that can have significant systemic health benefits.
OSA is a medical diagnosis that must be made by a physician or other suitably trained individual. From a medical legal perspective, an informed consent document is required to confirm that the patient is aware they are being treated provisionally, with the understanding that their condition entails medical risks for which they should consult with a physician.

With this signed consent, dentists can proceed with selecting an appliance. All PMAD appliances employ essentially the same mechanism of action:
Final appliance selection often comes down to clinician or patient preference, connector style, and price.
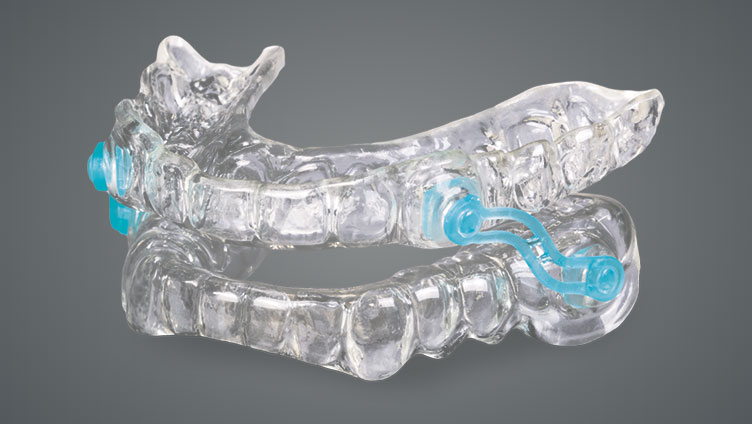
Helping patients achieve a deeper, more satisfying night’s sleep, the Silent Nite® Sleep Appliance treats snoring and mild to moderate sleep apnea quickly and effectively.
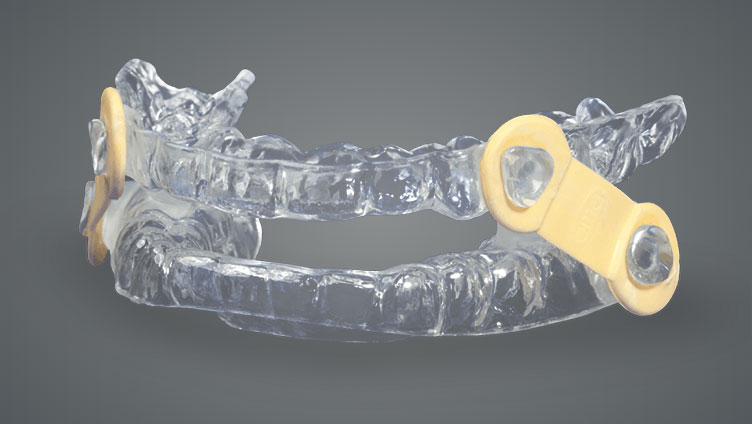
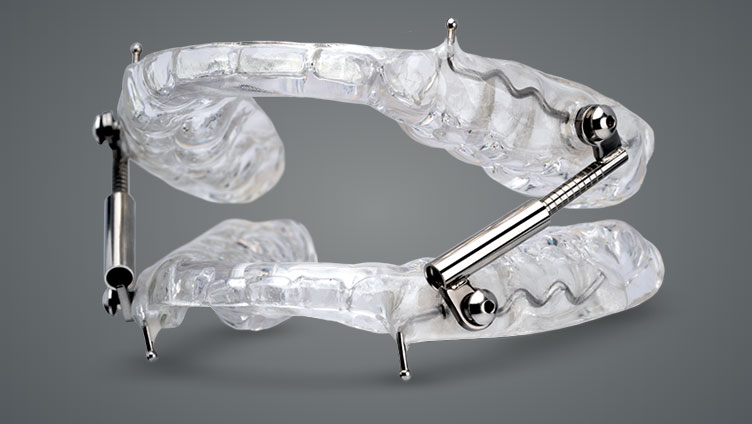
Designed to gently shift the lower jaw forward during sleep, the OASYS Hinge Appliance™ treats patients suffering from snoring and mild to moderate obstructive sleep apnea.
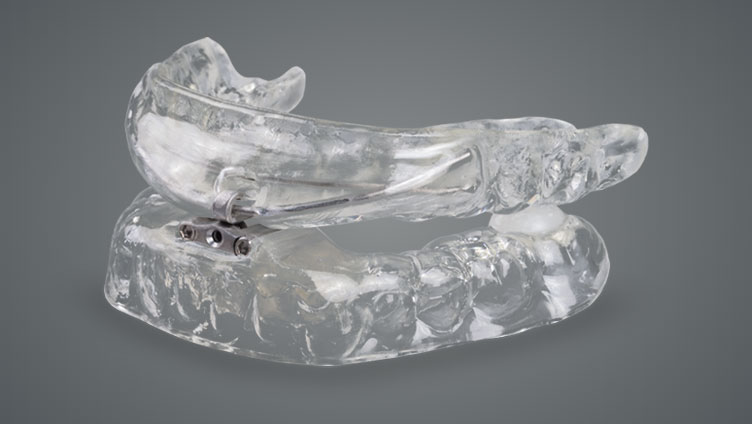
Developed with advanced technology, the dreamTAP™ appliance is designed to treat snoring and obstructive sleep apnea.
For any of the above oral appliances, take a bite registration with upper and lower impressions. Submit these with your Rx form to Glidewell.

Dental insurance does not pay for sleep appliances. Medical insurance requires a different mindset and can be confusing. The simplest, surest way to ensure compensation is to charge the patient directly for the provisional appliance and related chairside services.